Introduction: Immune cytopenias are a rare but potentially serious complication of immunotherapy (IO) used in the treatment of cancer. Immunotherapy-associated Immune Thrombocytopenia (IO-ITP) is a diagnosis of exclusion and often under recognized in the setting of cancer-directed multidrug regimens. In general it is managed with interruption of IO and disease-specific therapies based on management of the corresponding primary hematologic disorder. Our goal was to identify and characterize IO-ITP and to compare treatment approaches with primary immune thrombocytopenia (p-ITP).
Methods: Data was obtained from TriNetX, a de-identified national health research network with data sourced from 76 health care organizations (HCOs) with over 107 million patients located in the United States. We identified all adults (≥18 years) with diagnosis of ITP (ICD-10: D69.3) between 2015-2023. We categorized patients into two cohorts: IO-ITP, those with a diagnosis of ITP after IO use (pembrolizumab, nivolumab, atezolizumab, avelumab, durvalumab, ipilimumab) and a diagnosis of neoplasm (ICD-10: C00-D49); and p-ITP: those with ITP without neoplasm nor IO use. For both cohorts, we collected demographic, laboratory and treatment characteristics. To compare treatment approaches, we subcategorized cohorts by platelet count to identify patients who would qualify for ITP-directed therapy, using a platelet count ≤75x10 3/µL in IO-ITP and ≤50x10 3/µL in p-ITP. Platelet count cutoffs were defined based on current guidelines recommendations. We investigated the use of first-line and rescue therapies: glucocorticoids and IVIG; second-line therapies: thrombopoietin agonists (TPO-RAs: romiplostim and eltrombopag) and rituximab; third-line therapies: cyclophosphamide, azathioprine, cyclosporine, mycophenolate mofetil, bortezomib, avatrombopag and fostamatinib; and splenectomy, based on guidelines for management of p-ITP. For continuous data, we performed independent t-tests and for categorical data, chi-square tests. All tests were two-tailed with alpha level of .05.
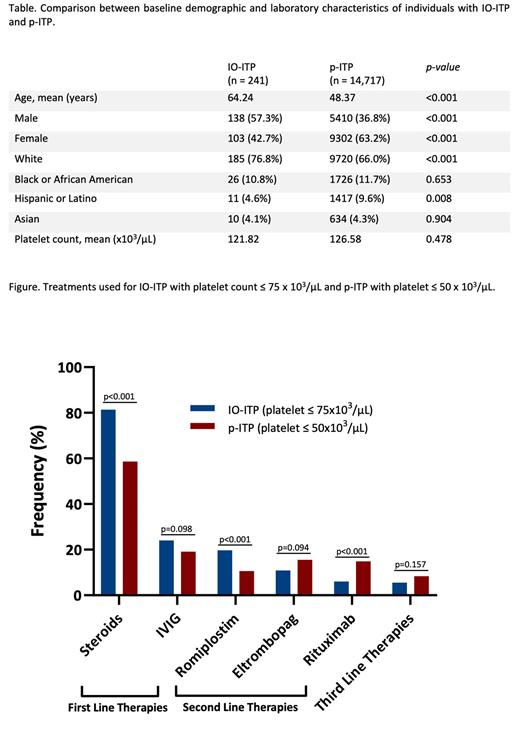
Results: In total, we identified 241 individuals with IO-ITP and 14,717 with p-ITP. Individuals with IO-ITP had a mean age of 64 years (21-86) and were predominantly male (57.3%), whereas those with p-ITP, were younger, (mean age 48 years,18-89) and predominantly female (63.2%) (Table). Both groups were mostly white and had similar mean platelet counts. Cancer of the respiratory system (40.7%) followed by skin malignancies (26.1%) were the most common subtypes in those with IO-ITP. Pembrolizumab was the IO most frequently used (52.7%).
Within first-line and rescue therapies, a higher proportion of patients with IO-ITP required steroids as compared to those with p-ITP (81.4% vs 58.6%, p value <0.001). There was no significant difference between the groups in the utilization of IVIG. With respect to second-line therapies, in IO-ITP, a greater proportion received TPO-RAs (27.9%) compared to rituximab (6%). More patients with p-ITP received rituximab as compared to IO-ITP (14.9% vs 6%, p value < 0.001). Among TPO-RAs, romiplostim was more commonly used in IO-ITP (19.7% vs 10.9%), whereas eltrombopag was preferred in p-ITP (15.5% vs 10.6%). Similar to p-ITP, the use of third line therapies was infrequent in IO-ITP. No patients with IO-ITP underwent splenectomy, which was also rarely used in p-ITP.
Conclusions: Our study describes treatment patterns in IO-ITP, an uncommon but challenging disease. We found that individuals with IO-ITP are generally younger and predominantly male as compared to p-ITP, highlighting different patient profiles. We further demonstrated some distinctions in the management of these two conditions, possibly suggesting variable response to therapy. Prospective studies are needed to better understand the determinants of such therapeutic choices and outcomes.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal